c-Met protein overexpression is distinct from MET gene biomarkers: it is defined by the cell surface protein, not a genetic aberration, so it is only detectable by IHC, not NGS or FISH1,4
MET IHC Testing

MET IHC testing can help identify patients for EMRELIS treatment


IHC is an established testing modality in NSCLC, and the VENTANA MET (SP44) RxDx Assay is a companion diagnostic to identify NSCLC patients with high c-Met protein overexpression1,3,4,6
IHC is also used to detect expression of protein biomarkers such as PD-L15,6
Turnaround time can be rapid compared with NGS testing17
Eligibility for EMRELIS treatment includes high c-Met protein overexpression, defined as ≥50% of tumor cells with strong (3+) staining, as determined by the VENTANA MET (SP44) RxDx Assay1,3†‡
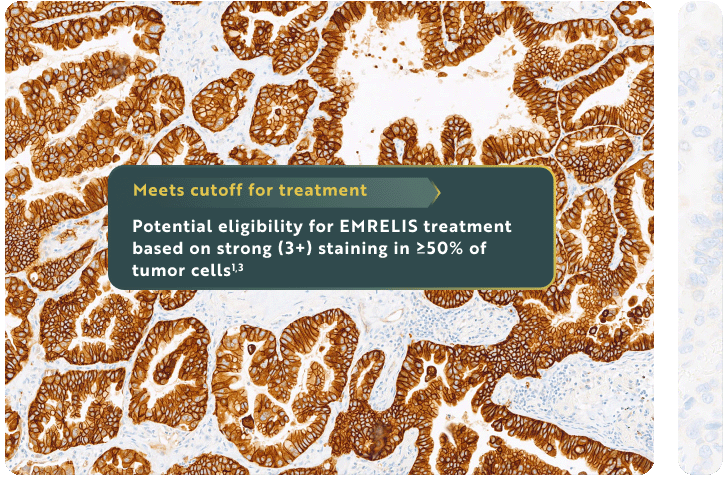
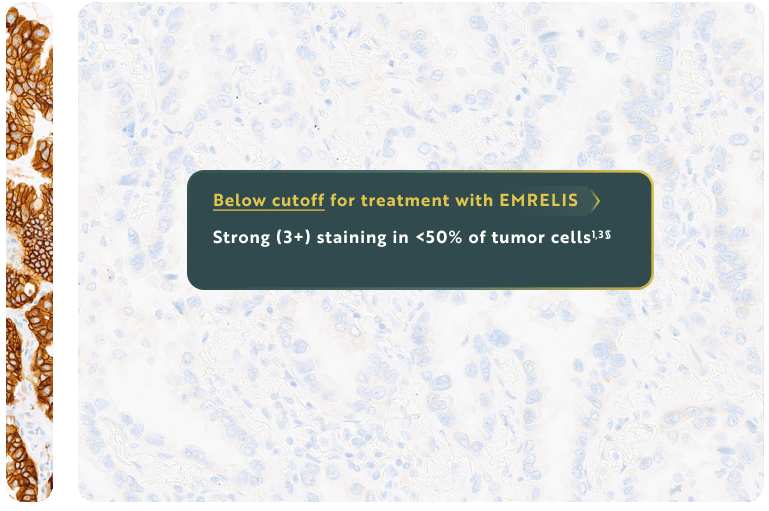
For illustrative purposes only.
Images provided by Roche Diagnostics.

THE VENTANA MET (SP44) RxDx Assay is a companion diagnostic to identify NSCLC patients with high c-Met protein overexpression1,3
Visit go.roche.com/MET to learn more about the VENTANA MET (SP44) RxDx Assay or download an Interpretation Guide.
See a list of labs that may offer the VENTANA MET (SP44) RxDx Assay.
You can test existing patients with archived tissue samples||

c-Met expression has been shown to remain consistent throughout the advanced/metastatic NSCLC disease course.10

Testing can be performed at diagnosis or thereafter (with archived tissue or re-biopsy).2||

In pathology report, look for
high c-Met protein overexpression to identify patients potentially eligible for EMRELIS treatment.1
VENTANA MET (SP44) RxDx Assay is indicated as an aid in identifying NSCLC patients eligible for treatment with EMRELIS for the indication and MET cutoff in accordance with the approved therapeutic product labeling. For more information, please refer to the official FDA approval and product labeling.
*Prior to enrollment, high c-Met protein overexpression, defined for patients with NSq NSCLC as ≥50% of tumor cells with strong (3+) staining intensity, was confirmed in archived or post-progression/recent sample at a central laboratory.1
†Confirmed in archived or post-progression/recent sample of at least 100 viable tumor cells.1,3
‡Defined as strong membranous and/or cytoplasmic tumor cell staining (3+) by the VENTANA MET (SP44) RxDx Assay.3
§IHC 1+ and 2+ staining intensities are considered below the cutoff for treatment with EMRELIS.1
||Screening samples for patients enrolled in LUMINOSITY were collected from diagnosis through post-progression.2
Next:
